An Arrhenius base is any species that increases the concentration of in aqueous solution. It produces a relatively small number of hydronium ions.
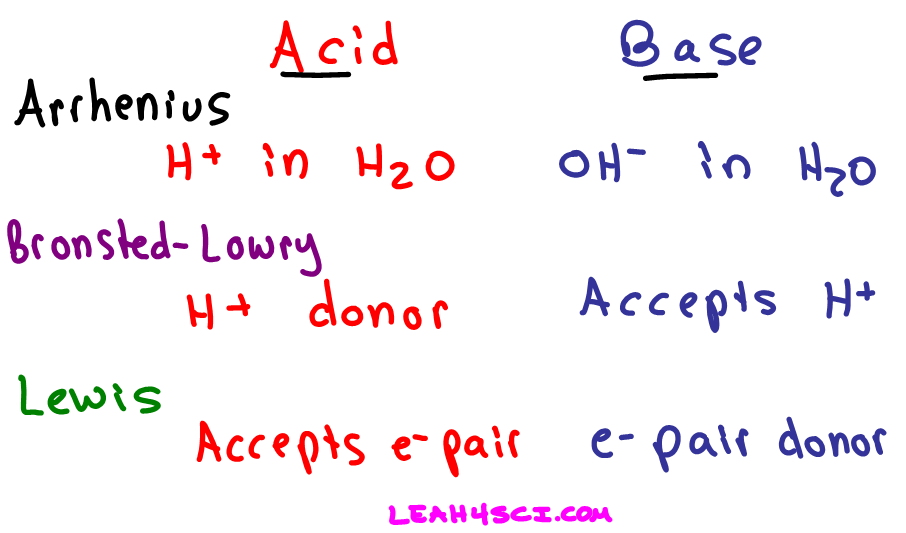
Arrhenius Bronsted Lowry And Lewis Acids And Bases In Organic Chemistry Mcat And Organic Chemistry Study Guides Videos Cheat Sheets Tutoring And More
Citric acid is best described as a.

. Soap toothpaste bleach cleaning agents limewater. Who are the experts. A neutralization reaction is a reaction between a base and an acid.
HCLO3 NH3 - NH4 ClO3-. Using the Arrhenius definition of acids and bases identify the Arrhenius acid and base in each of the following reactions. The Substance can be referred as Arrhenius Acid.
Strong acids and bases will be strong electrolytes. Both theories easily describe the first reaction. Bases H 2 O and SO 4 2-.
C3H85 02 3 CO₂ 4H₂O a If you begin with 35 moles of C3H8 and 62 moles of O₂. Find the moles of carbon dioxide produced_ b Find the mass of CO₂ produced using your work in step a above. Hydroxide is an OH- dissolved in water.
Which of the following does not describe an Arrhenius or Bronsted Lowry acid or base. View the full answer. Determine the pH of the buffer solution.
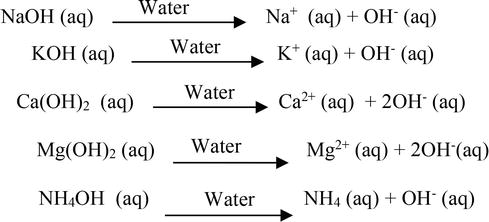
7 rows NaOH aq Na aq OH aq Some other examples of Arrhenius base are 1st and 2nd group. Question 7 of 25 Which of the following best describes an Arrhenius acid-base reaction. Which statement describes the Arrhenius interpretation of acids and bases.
The products are a salt and water. A temperature scale that never quite caught on was formulated by the. It is limited to situations that involve aqueous solutions or specific compounds.
Acid base H OH- C Acid base salt water D. Experts are tested by Chegg as specialists in their subject area. In an acid-base or neutralization reaction an Arrhenius acid.
In an aqueous solution an Arrhenius acid raises the concentration of hydrogen H ions while an Arrhenius base raises the concentration of hydroxide OH ions. In aqueous solution ions immediately react with water molecules to form hydronium ions. We review their content and use your feedback to keep.
Test your knowledge on arrhenius acid. Arrhenius Acids present in the equation are HCl HNO3 NH3 b. A buffer solution is prepared by mixing 100 L of 0050 M pentafluorobenzoic acid C6F5COOH and 100 L of 0060 M sodium pentafluorobenzoate NaC6F5COO.
The Ka of this weak acid is 0033. Label the Brønsted acids and bases in the following reaction. Acids HSO 4 - and H 3 O.
In a Bronsted Lowry acid-base reaction what are transferred from one reactant to another. A general definition based on electron structure. Why the Arrhenius model of acids and bases does not include ammonia in solution as base.
Which of the following best describes an Arrhenius acid-base reaction. An acidbase reaction is a chemical reaction that occurs between an acid and a base. Using the Arrhenius definition of acids and bases identify.
Arrhenius Acid Definition. This definition describes an acid as an oxide ion O 2. Acid base H20 B.
Acid base conjugate base conjugate acid. The acid-base reaction is considered a type of neutralization reaction where the acid and base react to yield water and a salt. With the Brønsted-Lowry theory being a subset of what acids and bases are and the Arrhenius theory being the most restrictive.
Hydroxide attacks and accepts the H from hydronium. Acidity and alkalinity describe the concentration of hydrogen ions acidity and hydroxide ions alkalinity. Hydronium breaks up to yield an H in solution.
Weak acids and bases will be weak electrolytes. Acid base salt water. As a result the interaction between an acid and a base in Arrhenius acidbase reactions is a neutralization reaction.
According to the Arrhenius description of acids and bases the water molecule consists of a proton and a hydroxide ion. Which is a base-conjugate acid pair. LiOH aqHNO3 aqLiNO3 aqH2O l CH32NH gHF g CH32NH2F s.
Given the following reaction answer the questions that follow. HSO 4 - aq H 2 Ol H 3 O aq SO 4 2- aq Answer. Acids and bases in aqueous solutions will conduct electricity because they contain dissolved ions.
Hydronium is an H donor regardless of solution. Therefore acids and bases are electrolytes. Which of the following would be classified as an acid according to the Arrhenius definition.
CH 3 COOH acts as an Arrhenius acid because it acts as a source of H 3 O when dissolved in water and it. When a Substance added to a water it will increase the H ions concentration. When a molecule is dissolved in water it.
Consider the reaction below.

Which Of The Following Best Describes An Arrhenius Acid Base Reaction Brainly Com

Follow Mxxnlightprincess For More Video Medical School Inspiration Science Notes School Organization Notes
0 Comments